Rall JM, Cox JM, Songer A, Comeaux JA, Estep JS, Cestero RF, Ross JD.
J Trauma Acute Care Surg 2013; 75(2 Suppl 2):S150-S156
In independent in-vivo test conducted, subjects treated with Celox (OMNI-STAT)* Gauze demonstrated the highest rate of observed survival with 90% when compared to the current standard of care, Combat Gauze demonstrated a 60% survival rate. Celox (OMNI-STAT) also has a substantial history of use on the battlefield (with conventional and special forces) and has repeatedly proven itself in austere settings.
Background: Uncontrolled hemorrhage is the leading cause of preventable death on the battlefield. The development, testing, and application of novel hemostatic dressings may lead to a reduction of prehospital mortality through enhanced point-of-injury hemostatic control. This study aimed to determine the efficacy of currently available hemostatic dressings as compared with the current Committee for Tactical Combat Casualty Care Guidelines standard of treatment for hemorrhage control (QuikClot Combat Gauze [QCG]).
Methods: The femoral artery of anesthetized Yorkshire pigs was isolated and punctured. Free bleeding was allowed to proceed for 45 seconds before packing of QCG, QuikClot Combat Gauze XL (QCX), Celox Trauma Gauze (CTG), Celox Gauze (CEL), or HemCon ChitoGauze (HCG), into the wound. After 3 minutes of applied, direct pressure, fluid resuscitation was administered to elevate and maintain a mean arterial pressure of 60 mm Hg or greater during the 150-minute observation time. Animal survival, hemostasis, and blood loss were measured as primary end points. Hemodynamic and physiologic parameters, along with markers of coagulation, were recorded and analyzed.
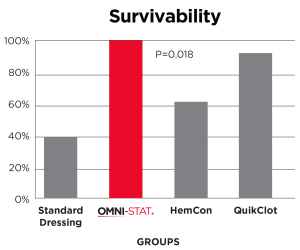
Results: Sixty percent of QCG-treated animals (controls) survived through the 150-minute observation period. QCX, CEL, and HCG were observed to have higher rates of survival in comparison to QCG (70%, 90%, and 70% respectively), although these results were not found to be of statistical significance in pairwise comparison to QCG. Immediate hemostasis was achieved in 30% of QCG applications, 80% of QCX, 70% of CEL, 60% of HCG, and 30% of CTG-treated animals. Posttreatment blood loss varied from an average of 64 mL/kg with CTG to 29 mL/kg with CEL, but no significant difference among groups was observed.
Conclusion: These results suggest that the novel hemostatic devices perform at least as well as the current Committee on Tactical Combat Casualty Care standard for point-of-injury hemorrhage control. Despite their different compositions and sizes, the lack of clear superiority of any agent suggests that contemporary hemostatic dressing technology has potentially reached a plateau for efficacy.

Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
Please leave us your details as below and submit or send us an e-mail to: info@omni-stat.com
This website uses Cookies to improve your browsing experience. View Cookies Policy
I'm fine with this